A press briefing from the RI Department of Health (RI DoH) COVID-19 vaccine team late in the afternoon on Wednesday, December 16, sounded like a victory lap: Everything was going to plan, the complicated series of deliveries of the Pfizer vaccine – so far the only one granted an Emergency Use Authorization (EUA) by the US Food and Drug Administration (FDA) – was arriving at hospitals, and nearly 500 high-risk, front-line healthcare workers had received the first of two required injections. Nearly 10,000 doses of the Pfizer vaccine were scheduled to arrive this week and another 10,000 doses were scheduled to arrive next week. If the FDA approves an EUA for the Moderna vaccine later this week as expected, about 19,000 doses would arrive next week.
UPDATE: The FDA approved an EUA for the Moderna vaccine late Friday night, December 18. An emergency meeting of the RI DoH Vaccine Sub-Committee is expected to be scheduled imminently, probably Monday morning.
Tricia Washburn of RI DoH said at the press briefing, “As of the end of day yesterday [Tuesday] in Rhode Island, we had received 4,875 doses of the Pfizer vaccine: of that 2,925 were received by the Lifespan healthcare system among Rhode Island Hospital, Miriam Hospital and Newport. And then the remaining doses were received, 1,950 doses received by the New England healthcare system among Kent Hospital and Woman and Infants hospital. And as of yesterday, the total number of front-line healthcare workers that were vaccinated was 489. We’re thrilled at the progress that we are making.”
The “pause” in RI, now expected to last three weeks, is working, as all leading metrics – percent positive in testing, new hospital admissions, and new cases per capita – have decreased this week compared to last week. As of December 17, the CDC reported that RI, recently in worst place for per capita daily cases, had improved to third place (behind Tennessee and Oklahoma).
Vaccine Shortages
The next day, Thursday, December 17, a late afternoon statement was issued by Gov. Gina Raimondo that, shortly after the Wednesday press briefing, the state had been informed by the federal government “the state’s allocation of the Pfizer vaccine for Week 2, beginning 12/21, was reduced from 10,725 to 6,825 doses. We have heard accounts of similar reductions in other states, and no clear explanation has been provided by Operation Warp Speed.”
As we previously reported, almost all of the second week supply of Pfizer vaccine in RI was allocated for nursing homes. Mckenzie Morton, the meeting facilitator for the RI DoH Vaccine Sub-Committee, told Motif after their regular weekly session on Friday, December 18, “While there was a change in vaccine supply, this did not impact our prioritization or distribution strategy.” Nursing homes and other long-term care facilities were scheduled to receive 50% of their needed supply next week and the remaining 50% the following week, but because of the decreased supply will instead receive 25% each of the next four weeks. “This additional flexibility has allowed us to stay on course with our plan while not disrupting the plan to vaccinate nursing home staff and residents,” she said. The order in which nursing home residents and staff will be visited by teams from CVS and Walgreens, the contractors chosen by the federal government for the task, will not change, concentrating on where the virus presents most risk due to prevalence. “The prioritization by geography and other factors has been a mainstay of our plan since day one due to RI’s focus on equity as a principle that guides all of our decisions,” she said.
The company issued a statement saying, “Pfizer is not having any production issues with our COVID-19 vaccine, and no shipments containing the vaccine are on hold or delayed. This week, we successfully shipped all 2.9 million doses that we were asked to ship by the US Government to the locations specified by them. We have millions more doses sitting in our warehouse but, as of now, we have not received any shipment instructions for additional doses.”
The unexpected decrease in allocation led to a round of finger-pointing between Pfizer and the federal government, according to numerous news sources, including Bloomberg and The New York Times. According to both, the immediate cause of the confusion was the federal government changing their day of allocation from Friday to Tuesday in response to pleas from states to get information earlier, leading to allocations using earlier and therefore smaller numbers, effectively delaying shipment from warehouses by one week. According to Bloomberg, the federal strategy of holding back supply for second required second doses also plays a role.
Raimondo continued, “We are calling on the Trump administration to honor its commitments and provide the full allocation to Rhode Island. In the meantime, we are continuing to distribute the vaccine as quickly as possible to our frontline healthcare workers, and we are evaluating the impact of these reductions on our vaccination planning.”
Priority groups
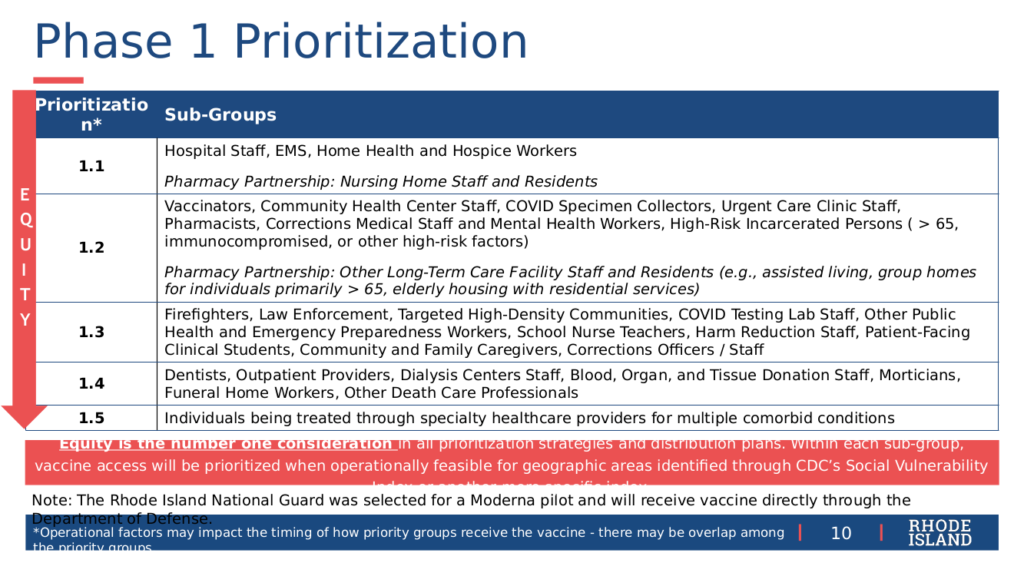
The main topic of the RI DoH Vaccine Sub-Committee at their regular meeting on Friday, December 18, was finalizing a recommendation for the criteria for Phase 1 of the vaccination effort. Due to the limited supply of vaccine and resources to administer it, Phase 1 is expected to take a minimum of three months. Under the recommended criteria, an estimated 150,000 people – 15% of the state population – will be eligible within the five subgroups named in order of decreasing priority as Phases 1.1 through 1.5. Within subphases 1.1 and 1.2, the plan distinguishes those who will be vaccinated directly under the state program and those who will be vaccinated by pharmacy contract partners (that is, CVS and Walgreens). Contractors are expected to visit 295 sites, according to Alysia Mihalakos of RI DoH at the Wednesday briefing.
In Phase 1.1, hospital staff, EMS, home-health and hospice workers will be vaccinated directly, while nursing home staff and residents will be vaccinated by contractors. In Phase 1.2, vaccinators, community healthcare staff, COVID-19 specimen collectors, urgent care clinic staff, pharmacists, corrections medical and mental health workers, and high-risk incarcerated persons (over age 65, immuno-compromised, or other high-risk factors) will be vaccinated directly, while other long-term care facility staff and residents (assisted living, group homes, elderly housing with residential services) will be vaccinated by contractors.
Phase 1.3 covers firefighters; law enforcement; targeted high-density communities; COVID-19 testing lab staff; public health and emergency preparedness workers; school nurse teachers; harm reduction staff; patient-facing clinical students; community and family caregivers, and corrections officers and staff. Phase 1.4 covers dentists; outpatient providers; dialysis center staff; staff handling donations of blood, organs, or tissue; morticians, death care, and funeral home workers. Phase 1.5 covers individuals being treated through specialty health care providers for multiple co-morbid conditions.
Pregnancy and Breastfeeding
Considerable attention has been accorded to the advice that women who are or may be pregnant, or who are breastfeeding, should consult with their PCP in deciding whether to be vaccinated, but what advice should be given to PCPs how to counsel patients?
At the Wednesday press briefing, Pablo Rodriguez made attendees aware of a formal practice advisory just released from the American College of Obstetricians and Gynecologists – acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19 – which states, “Important considerations include: the level of activity of the virus in the community; the potential efficacy of the vaccine; the risk and potential severity of maternal disease, including the effects of disease on the fetus and newborn; the safety of the vaccine for the pregnant patient and the fetus.” The statement continues, “Vaccines currently available under EUA have not been tested in pregnant women. Therefore, there are no safety data specific to use in pregnancy… Pregnancy testing should not be a requirement prior to receiving Pfizer-BioNTech vaccine. Pregnant patients who decline vaccination should be supported in their decision.”
An article published December 17 in The Lancet, generally regarded as the most prestigious medical journal in the UK, noted that the lack of data about drug safety in pregnancy is a result of 75% – 80% of studies excluding pregnant women by protocol, even in relatively low-risk innocuous situations: “Of 35 studies that evaluated high-dose vitamin treatment for COVID-19, 27 (77%) excluded pregnant women.”
At the Wednesday press briefing, Philip Chan of RI DoH and Brown Medical School answered a question from Motif about how to advise pregnant patients in considerable detail, giving practical advice. “First off, I do want to say that the studies are in progress. So as we every single week, every month here, is that we are going to have more and more accumulating data certainly about the vaccine and pregnancy. Certainly as people start to get vaccinated, we’re going to have a whole lot of information in the near future.”
Chan continued, “What I do when I talk to my patients about things like this, I have to look at the person, I have to understand who the patient is in front of me. I have to understand what their risk is of COVID-19. So I have some colleagues that are pregnant, who are testing and treating COVID-19 in the hospital. So to me, that’s a really high-risk situation, and I would be more likely to encourage someone in that situation to get the vaccine. If there’s an average Rhode Islander who is at home or working from home, pregnant, who’s not coming into known contact with COVID-19 on a daily basis, I may be more likely to tell them to hold off on the time being until we receive more data. So that’s why it’s a little bit more of this nuanced individual discussion: You want to take into account, obviously, a person’s values, obviously hear what their concerns are. But as a physician, you also want to take into account their exposures in order to best best guide them. So that’s how I think about it, and that’s why we’re sort of encouraging people to have an individual level discussion with their primary care provider.”
In response to a follow-up question from Motif whether there was any evidence that pregnant and breastfeeding women should not be vaccinated, Chan said there was not. “If we – the scientific community, public health physician community – if we had any reasons to suspect this vaccine was a danger to individuals that were pregnant or breastfeeding, then absolutely we would not recommend it. But your point is, there’s no evidence and there’s no biological indications or thought process that it would be damaging, which is why, in general, the CDC [and] we feel comfortable at least offering it to pregnant women, especially because of some of the potential complications that can arise from COVID-19 and pregnancy.”
Equity of Access
In response to a question from Motif about equity of access at the Wednesday briefing, especially to contractor-provided vaccinations, reaching both nursing home residents and staff who may have significant disabilities, including cognitive disabilities, as well as limitations of English language and transportation, Mihalakos said, “We have asked [contractors] for the their language capability, and hope that, as they’re going through their recruitment process, that they are recruiting staff who are reflective of the communities that they’re going to be serving.”
Mihalakos said, “One-hundred percent of staff in those facilities will be offered the vaccine, and one of the challenges and one of the factors that we’re considering is that if – there is no finalized plan for this, I want to be really clear on this – but one of the things that we’re exploring is that if we offered more opportunities so that we can safely rotate staff so that they’re not all getting vaccinated at once… we want to ensure that the folks who who don’t have easy access to transportation are vaccinated on site and those who do have transportation are able to drive to a vaccination site. We want to make sure that people for whatever reasons are not blocked from the opportunity to be vaccinated. So as we learn more about what the opportunities are, we will work with the leadership of the nursing homes, and after that the assisted livings, the group homes, and the residential care facilities to ensure that they all know and understand that as well, so that when they’re making decisions about the staff. We don’t have the same concerns about the residents: all of the residents can get vaccinated at the same time, because we don’t have that same concern about protecting the protectors. But we want to ensure that all staff have absolute, unfettered access to getting vaccinated.”
In response to another question from Motif about outreach to home healthcare workers who would not be covered as nursing home staff, Mihalakos said, “We’ve begun the conversation with the administrators of the home health agencies to let them know what our expectations are for the first round of available vaccination, which is via regional pod model. So at the same time the municipalities will be activating those points of dispensing, we will be offering that opportunity up to all home health and hospice workers to ensure that they have access as well. It is imperfect because it is a regional model.”
Mihalakos continued, “We’ve been talking to the leadership of the trade organization that represents home care about this opportunity. We also queried all of the home health agencies to see who was willing to vaccinate both their own patients when we get to that phase and individuals with co-morbidities who may be covered by home care workers already, but also because we know that there are several large home health agencies in the state that already provide vaccination services to other home health agencies that are near them. So we may be able to leverage that opportunity with a dozen or more large home health agencies that have have raised their hands and said yes, they’re willing to help with this initiative. So we’re going to try to create the broadest coverage we can… We have pledged to then survey after we go through the first round to see what type of coverage we got so that we understand what may have been due to people’s either unwillingness to get vaccinated at the time or not having a level of interest of getting vaccinated at the time that they were offered, or if there were other barriers to them getting vaccinated so that we can create more targeted approaches.”



