Approval was granted this morning at an emergency meeting of the Vaccine Sub-Committee at the RI Department of Health (RI DoH), conducted virtually, to begin administering vaccines against COVID-19. According to Mckenzie Morton who acted as meeting facilitator, the vote was 95% in favor.
The supply of vaccine will be severely limited, and at this point, the state has been given no projections beyond the next two weeks, according to Alysia Mihalakos, the chief of the Center for Emergency Preparedness and Response at RI DoH. “It’s an amazingly exciting day. It was a whirlwind of activity all weekend long preparing for today,” she said. The Monday emergency meeting was the result of a call at 2pm Sunday, she said.
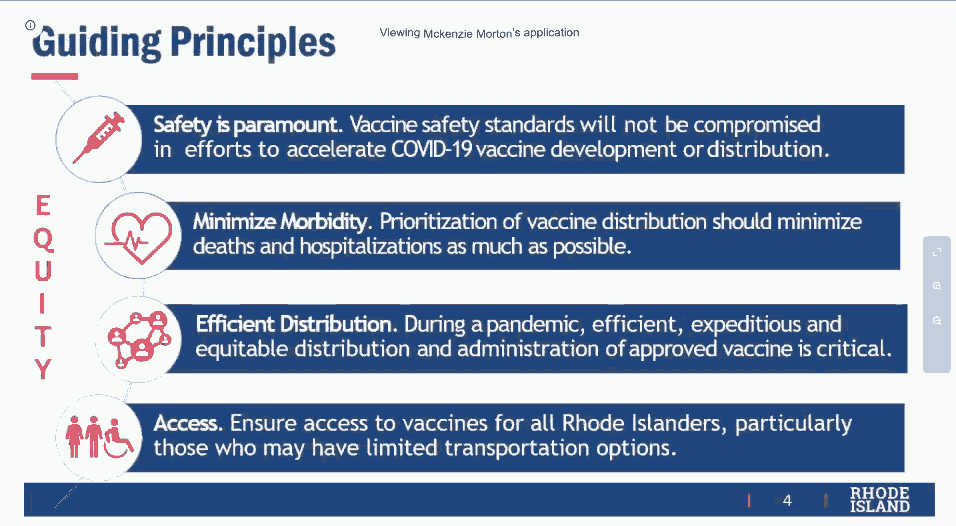
Early in the meeting, the “guiding principles” of the sub-committee were reviewed: “Safety is paramount,” “Minimize morbidity,” “Efficient distribution,” and “Access.”
The RI authorization allows “high-risk” front-line healthcare workers 16 years of age or older to be vaccinated. Each hospital or healthcare provider is responsible for defining and assessing “high-risk” in this context. Several different vaccines from different makers are in the approval pipeline, and so far the US Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA) to only the Pfizer vaccine this past Friday, December 11, but is expected to authorize the Moderna vaccine within the next few days. Two doses are required for full effectiveness, 21 days apart for the Pfizer vaccine and 28 days apart for the Moderna vaccine. In trials involving 30,000 volunteers each, both vaccines were shown to be 95% effective at preventing COVID-19 disease, an extremely high level of vaccine efficacy.
Nursing home residents were moved by the federal guidance into the top priority, Mihalakos said, “which shifted our operational planning.” The Pfizer vaccine, shipped in 975-dose batches, will arrive in RI with 9,750 doses this week and 10,725 doses next week, she said, which is consistent with what has been planned for the past two weeks. All of the doses this week and 975 of the doses next week are allocated to healthcare workers. Meeting approximately half the need requested, 9,750 of the doses next week are allocated to nursing homes to be distributed through CVS and Walgreens as partners with the state. RI also expects to receive “19,000-ish” doses of the Moderna vaccine within the next two weeks, she said, assuming it follows a smooth approval process similar to the Pfizer vaccine. Vaccine shipments will be arriving today (Monday) at RI Hospital and Newport Hospital; tomorrow (Tuesday) at Kent County Memorial Hospital, Women and Infants Hospital, and Miriam Hospital; then Thursday at Landmark Medical Center, South County Hospital, and Roger Williams Hospital. Much of the initial allocations reflect logistical constraints because the Pfizer vaccine is shipped in such large batches and has to be stored in special freezers below -80°C. Roger Williams, which has the necessary freezer, will share with Our Lady of Fatima Hospital, which doesn’t. Bradley and Butler hospitals will be covered by their parent organizations this week, but are expected to get direct shipments next week of the Moderna vaccine that has much less stringent storage requirements and is shipped in smaller batches. Westerly Hospital and Eleanor Slater Hospital, because they have fewer staff, will get their vaccines through the redistribution system used for nursing homes.

Considerable discussion at the meeting concerned special situations, such as whether pregnant or breastfeeding women should be vaccinated. Guidance from the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC) is that this is a decision for individual patients to make in consultation with their regular physician, taking into account their risk of exposure and risk of bad outcome from pre-exisiting underlying conditions, but several sub-committee members expressed concern with the practical consequences of that advice. Will Giordano-Perez said, “I think it’s important to notify people that they have the opportunity to talk to their PCP, however, we’ve not prepared at all any of our PCPs in this state to really discuss, I think, at length and give recommendations as far as whether or not someone is prepared, or should, or is a candidate for vaccination. I just want to be mindful of that because I know a lot of the PCPs are just going to be like, ‘I have absolutely no idea. Should you get it? I don’t know.’” He continued, “I think that could be a really big burden on PCPs, especially if they’re not properly prepared or haven’t had an opportunity to review the data. I think it will be important whatever we decide here.” Pablo Rodriguez echoed his concern, saying he had been in touch with OB/GYN practitioners and midwives who feel “completely up in the air” as they are waiting for clear CDC guidance. “The statement in the [FDA] Emergency Use Authorization is ‘There is inadequate data on pregnant and breastfeeding women to assess the safety and efficacy of the vaccine,’ so we understand that is going to be a unique challenge for which there is not a strong answer,” Mihalakos said.
Utpala Bandy stressed the importance of providing advice to physicians, noting with regard to known side effects of vaccination, “The main concern is fever, because pregnant women and fever are not a good mix. And fever has been associated with adverse pregnancy outcomes, especially early pregnancy, so the simple answer to that is they should be counseled to be taking Tylenol and that routine testing for pregnancy prior to receipt of the COVID vaccine is not recommended.” She said, “These kinds of non-live vaccines are routinely given to pregnant women, anyway, but not the mRNA, which is a little more innocuous than the [vaccines] we already give them, such as pertussis, such as influenza.” (Both the Pfizer and Moderna vaccines are mRNA-based, containing no virus particles at all.)
Mihalakos reviewed the federal guidelines for vaccination: patients who previously had COVID-19 infections should be vaccinated, although those treated with monoclonal antibodies or convalescent plasma should wait 90 days after treatment; patients who received any other vaccine should wait 14 days; patients with active and symptomatic COVID-19 infection should wait until symptoms subside and at least 10 days after ending isolation. Patients with a history of severe allergic reaction (that is, anaphylaxis) to vaccination should be individually evaluated, and should be monitored for 30 minutes following vaccination; provided that patients with known allergies to specific ingredients in the vaccine (including lipid nanoparticles and polyethylene glycol) should not be vaccinated. Patients with general allergies (food, pets, insects, environmental, latex, oral medication) or a family but not personal history of anaphylaxis can be vaccinated.
Several members, including Giordano-Perez, Joan Kwiatowski, and Chris Abulime, said they wanted to see assurances of equity in vaccine distribution, with outreach to the minority community. Larry Warner expressed a concern about information being made available to visually impaired and non-English speaking patients. “The CDC has pledged to put everything out in 25 languages, so we’re hoping they will do some of the heavy lifting for us,” Mihalakos said, assuring him that this issue would be addressed. Tricia Washburn of DoH cautioned that such issues were beyond the scope of today’s emergency meeting, which is restricted by the state Open Meetings law, but would be discussed at the regular meeting this coming Friday, December 18, along with broader operational matters.
“After a rigorous scientific review, we know that COVID-19 vaccine is safe. We also know that it is one of the most effective vaccines ever developed,” said RI DoH Director Nicole Alexander-Scott, in a statement. “In the coming weeks and months, as vaccine becomes more available, getting vaccinated will be one of the most powerful things you can do to keep yourself and the people you love safe from COVID-19. We are going to work to ensure that every person in every community in Rhode Island has access to the vaccine, especially those communities hardest hit by this virus.”
“We have never had a vaccine that has been – or will be – more closely monitored than the COVID-19 vaccine,” said Philip Chan, consultant medical director for the RI DoH Division of Preparedness, Response, Infectious Disease, and Emergency Medical Services, in the same statement. “Teams of scientists at the national level have been scrutinizing thousands of pages of technical data for weeks, focusing on vaccine effectiveness, safety, and the manufacturing process, and our own local review has happened here in Rhode Island. I absolutely plan on getting vaccinated when it is my turn.”
