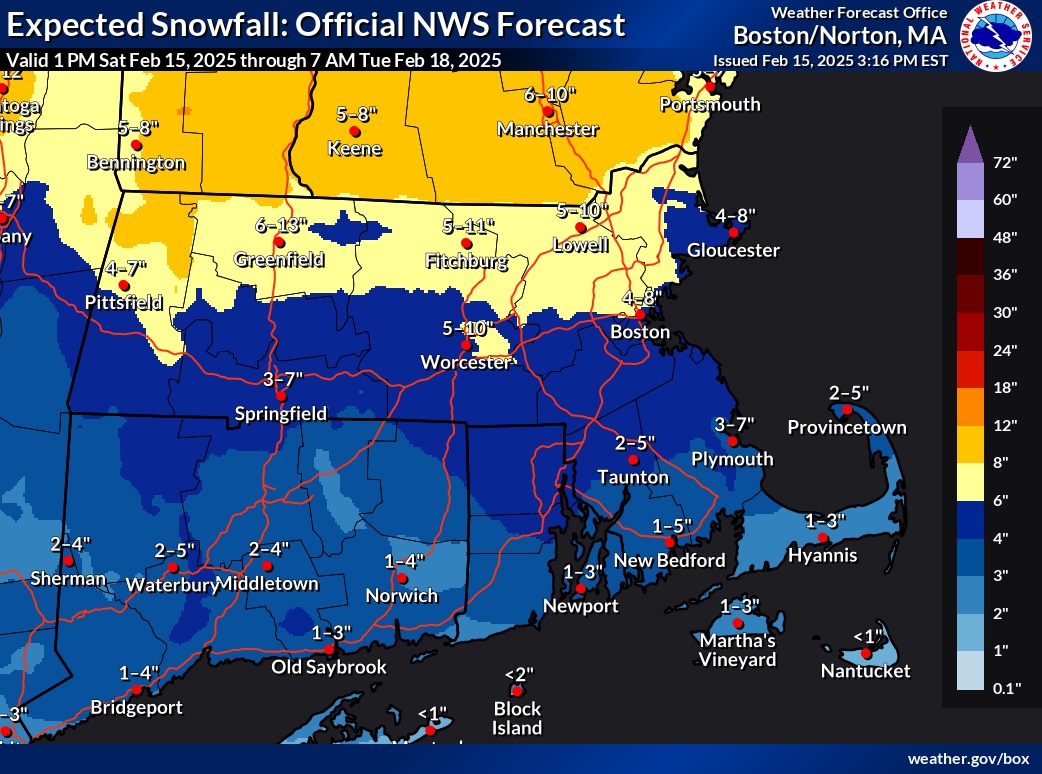
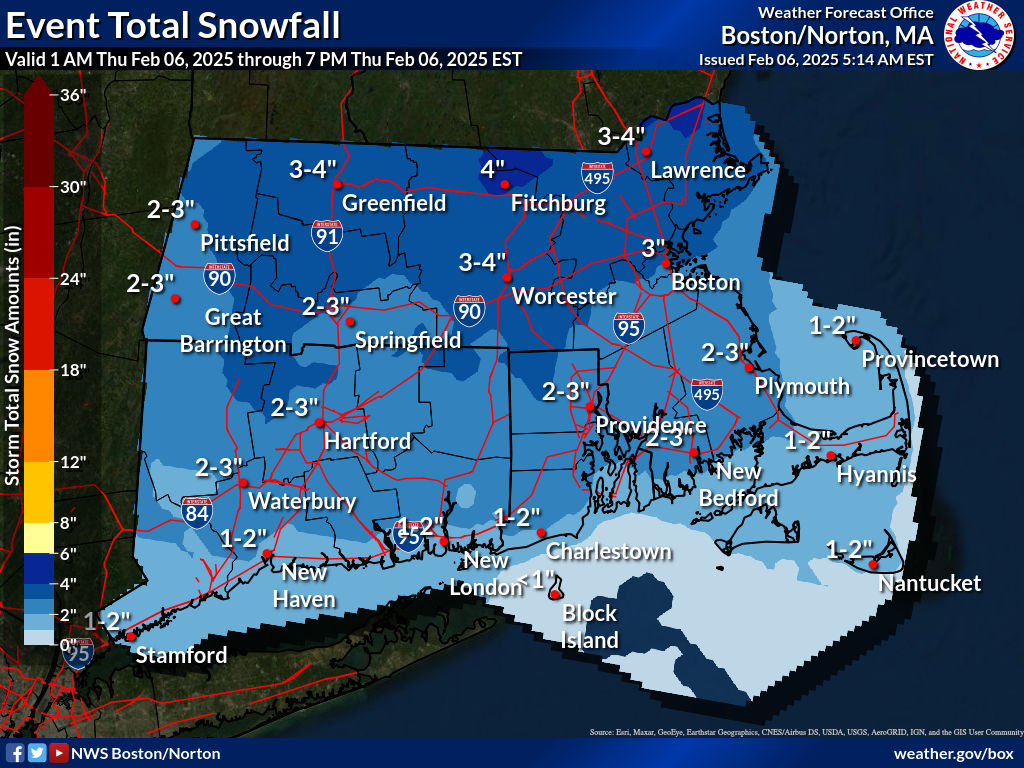
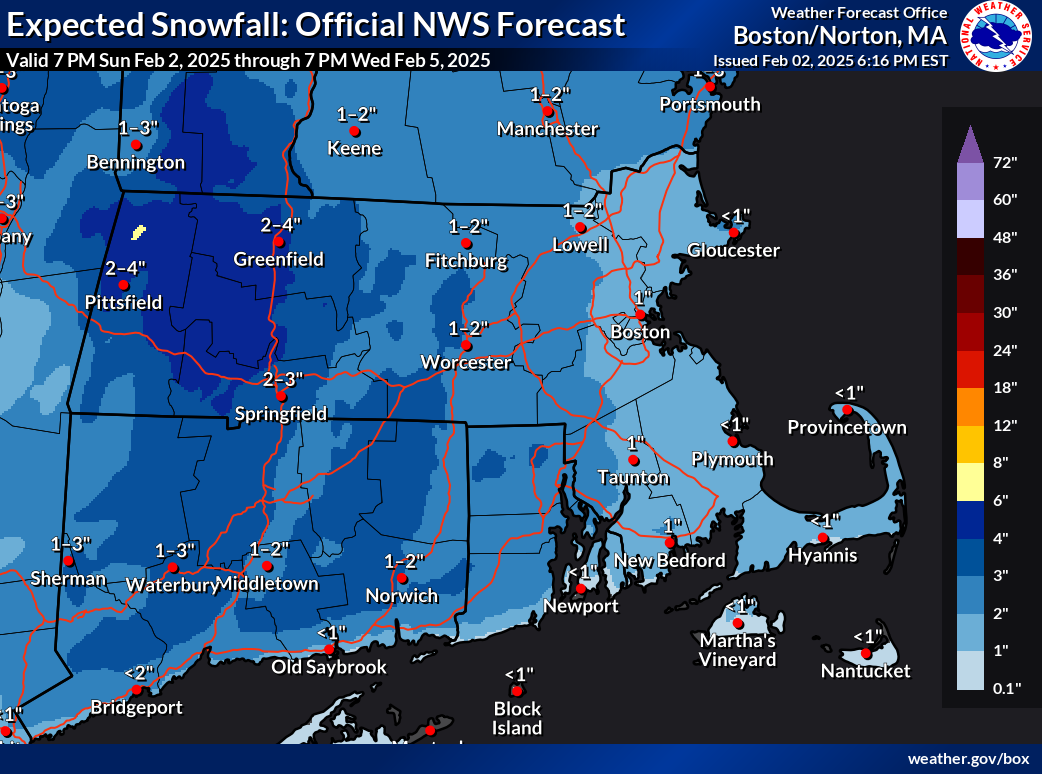
Opinion — PVD City Council Flies Palestinian Flag
Check your privilege while globalizing the intifada
The members of the PVD City Council who asked on May 14 to fly a Palestinian Arab flag in their chamber and in front of City Hall, and held a […]