
The Moderna vaccine against COVID-19 was unanimously approved for use in RI this morning at an emergency meeting of the RI Department of Health (DoH) Vaccine Sub-Committee, following the issuance by the US Food and Drug Administration (FDA) of an Emergency Use Authorization (EUA) on Friday, December 18. Doses are expected to begin arriving in RI later today, a planned total of 19,000 over the next week, according to Alysia Mihalakos of DoH.
The Pfizer vaccine was previously approved for use in RI on Monday, December 14, and 9,750 doses were received last week, but in the second week of distribution beginning today the state was warned by the federal government to expect only 6,825 instead of the scheduled 10,725 doses.
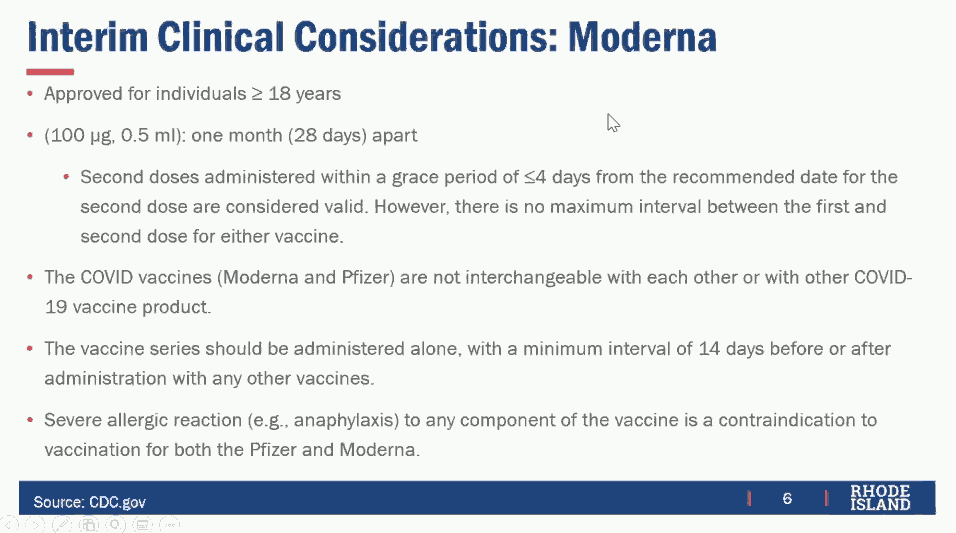
The two vaccines are very similar and both are based on mRNA technology, but there are some important differences. The Pfizer vaccine is approved for patients age 16 and older, while the Moderna vaccine is approved for patients age 18 and older. Both require two doses for full immunity, but the Pfizer vaccine recommends 21 days between doses and the Moderna vaccine recommends 28 days between doses. (Although it is recommended that the second dose be given as soon as allowed, neither vaccine has a maximum interval between doses, so giving the second dose late is better than not giving it all.) While the Moderna vaccine requires cold storage and transportation, it is less demanding than the Pfizer vaccine that requires extremely cold temperatures below -80°C. The vaccines cannot be combined or interchanged, such as by giving a first dose of one and a second dose of the other. Neither vaccine should be given to anyone with a known allergy to any of its ingredients.
In response to a question from Motif, sub-committee member Pablo Rodriguez confirmed that patients who are pregnant or lactating should follow the same procedures for both vaccines, which is to consult with their PCP for an independent evaluation of risk factors.
In response to questions from Motif, Mihalakos said that despite the unexpected shortfall in doses of Pfizer vaccine, the Moderna supply is as expected. “We are on track to receive our 19,000 this week, so there have been no changes overall in the planning estimates that you may have heard about previously. The vaccine will be distributed to all hospitals because we have not received nearly enough vaccine yet to cover all of the employees of all hospitals.” All of the first week supply of Pfizer vaccine, which was received last week, was allocated to hospitals to vaccinate their front-line staff, but the vast majority of the second week supply of Pfizer vaccine, nearly half of which is delayed, was allocated for federally contracted pharmacy partners, CVS and Walgreens, to vaccinate nursing home residents and staff, especially because the contractors are equipped to provide the extremely cold storage and handling needed. The plan was therefore to use the Moderna vaccine when possible primarily for hospital staff beginning this coming week. “We’re vaccinating the vaccinators, so our two mass-vaccinator partners received an initial supply of vaccine last week they will continue this week. We will be working with community health centers to ensure that their staff can start to be vaccinated. Again, we are moving on some plans for rolling vaccine because everybody can’t be vaccinated at once. There will be some additional vaccine that goes to the ACI to follow up on the initial doses they got this week,” Mihalakos said. “The Pfizer vaccine that we are allocated in this [coming] week, the majority of that will go to the pharmacy partnership for the beginning of the nursing home vaccinations. So you’ve heard us speak previously about that we needed to have 50% of the vaccine for staff and residents up-front.”
Much of the work of the sub-committee is highly dependent upon data and guidance supplied by the US Centers for Disease Control and Prevention (CDC), particularly its Advisory Committee on Immunization Practices (ACIP), which published clinical recommendations for the Moderna vaccine only yesterday in a special edition of the Morbidity and Mortality Weekly Report (MMWR), the principal journal of the CDC. RI sub-committee meeting facilitator Mckenzie Morgan, noting how well things were going, joked, “It’s really nice once in the past month to not get surprises this weekend, which was, I don’t know about you guys, but a welcome relief for me. And I’m sure the rest of the RI DoH team got a huge sigh of relief. When I read the MMWR, I was like, ‘Okay, nothing crazy. All right. Awesome.’”
The next regular meeting of the Vaccine Sub-Committee is scheduled for Friday, January 8, skipping the holiday week when the usual meeting day, Friday, would fall on New Year’s Day, but members were warned that emergency meetings may be called if needed.



