Children in RI could start receiving COVID-19 vaccinations on November 8, 2021, “give or take a few days,” Department of Health (DoH) staff member Tricia Washburn told a meeting of the DoH Vaccine Sub-Committee on October 27.
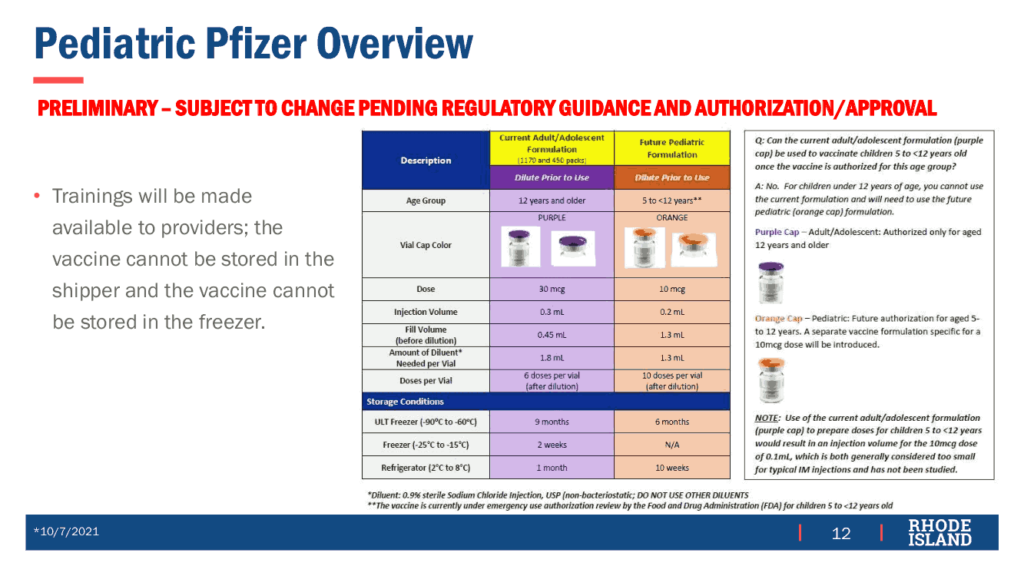
The prior day, the US Food and Drug Administration (FDA) Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted 17-0-1 to recommend authorization of a Pfizer vaccine for children. While the Pfizer adult vaccine had previously been authorized for age 12 and older, the vaccine for ages 5-11 is a new pediatric formulation with one-third the dose; data submitted by Pfizer showed it to be 90.7% effective in preventing symptomatic COVID-19. The pediatric formulation is color-coded in orange packaging to prevent confusion with the adult formulation in purple packaging.
Although the FDA usually follows the recommendation of its advisory panels of experts, it is not required to do so. UPDATE: Late in the afternoon of October 29, the FDA officially issued an emergency use authorization (EUA). UPDATE: Final CDC authorization was issued in the evening of November 2, and RI began vaccinating children on November 4.
While the FDA is a regulatory body that decides whether a drug is safe and effective, after FDA authorization it is the role of the US Centers for Disease Control and Prevention (CDC) to recommend appropriate use, and its Advisory Committee on Immunization Practices (ACIP) is scheduled to meet on November 2-3 to consider use of the pediatric vaccine, including for which patients it may or may not be appropriate.
Many of the VRBPAC members expressed concern that the data were based on small studies with an insufficient number of participants to detect rare adverse reactions that may occur less frequently than 1 in 10,000 vaccine administrations, thereby making cost-benefit analysis difficult because most children infected by COVID-19 have mild cases. Rare adverse reactions such as heart inflammation (myocarditis and pericarditis) can be painful and require hospitalization, but so far have resulted in fairly quick full recovery.
It is hoped that the lower-dose pediatric formulation will reduce risk of adverse reaction relative to the adult formulation. The consensus was that for some children, especially those with existing medical conditions that leave them at high risk for severe complications if infected, the benefit of vaccination is clear. For the average healthy child, however, while vaccination does protect the child being vaccinated, the main benefit of vaccination is to protect others and the community as a whole, raising a question of medical ethics as to whether it is appropriate to expose them to risk, however small, of adverse reactions. For this reason, the VRBPAC members cautioned, COVID-19 vaccine mandates or requirements for children would be premature, and they expressed the hope that the ACIP guidance would respect the rights of individual families to decide to defer vaccination for young children.
Assuming the FDA emergency use authorization (EUA) and CDC guidance are in place by November 4, Washburn said, state-run vaccination facilities, such as Sockanosset, could begin vaccinations for ages 5-11 around November 8.
“November 4 is the ‘best possible case,’ assuming CDC issues final guidance and any clinical recommendations by that date, and we have all admin operations for state sites ready to go,” Washburn told Motif via e-mail. Administrative work includes incorporating the FDA and CDC guidance into informed consent forms on the web registration system and translating this into multiple languages. “The team works pretty quickly and it is possible but I am very cautious with setting expectations, so that is why I said it could be sometime before or after November 8. There are some sites like pharmacies, doctor’s offices, hospitals and federally qualified health centers that are ready to start on Nov. 4, assuming CDC comes through by that date, as they run their own registration systems.”
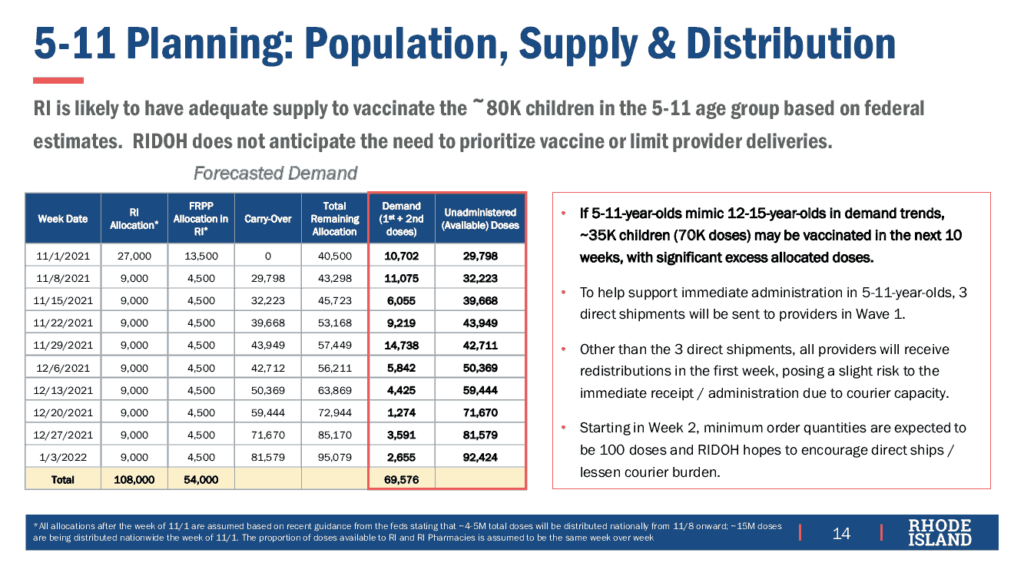
The state expects an initial allocation of 27,000 doses of the Pfizer pediatric vaccine in three tranches of 9,000 doses each, following the EUA first in 1-5 days, second in 3-7 days, and third in 5-9 days. After that first week, supply is expected to flow at 9,000 doses per week, making available a total of 108,000 doses by early January 2022. In addition to the doses supplied through the state, the Federal Retail Pharmacy Partnership (FRPP) is expected to receive an initial allocation of 13,500 doses in the first week after EUA and continuing to receive 4,500 doses each week after that, a total of 54,000 doses by early January 2022. Because each child requires two doses, supply will be sufficient to vaccinate 81,000 children, comprising the entire age 5-11 population of RI.
COVID-19 vaccinations are completely free to patients by federal government regulation, so parents who choose to have their children vaccinated by their regular pediatricians will be exempt from co-pays and other fees such as office visits if the only service provided is vaccination.
Several members of the Vaccine Sub-Committee expressed concerns that pediatric offices may be choosing not to participate in COVID-19 vaccination programs because it takes more time to vaccinate children than adults, especially if there is significant counseling involved to address cost-benefit concerns. While none suggested a desire to bill patients directly, they suggested the state work with insurers to create a separate billing code for the 15-30 minutes of counseling expected to accompany pediatric vaccination, a concern especially for clinics and practices serving large Medicaid populations where the economic operating factors are already at the margins.
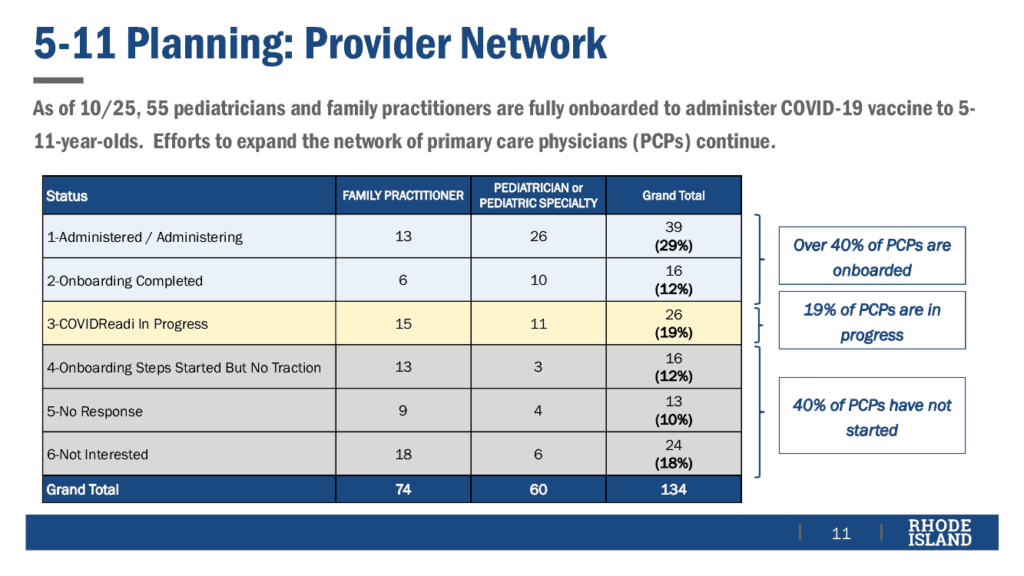
As of the meeting, of the 134 family and pediatric practices in RI, 40% are ready to administer vaccinations, 19% have started but not completed the process of being ready, and 40% have not started.
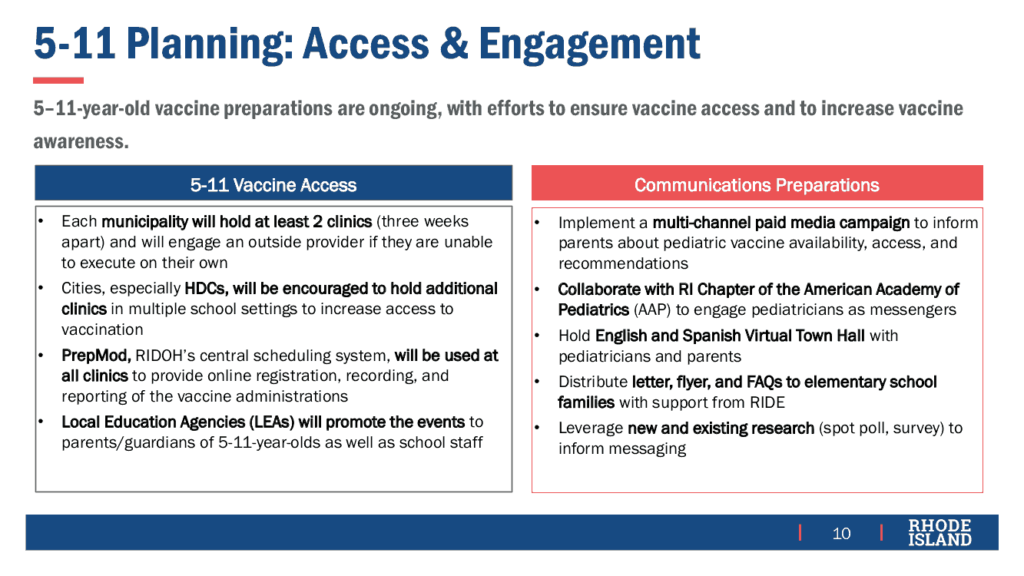
Each of the 39 cities and towns in RI will hold at least two pediatric vaccination clinics to administer first and second doses. Local education agencies (LEAs) will hold clinics in schools. High-density communities (HDCs), such as Central Falls, will hold additional clinics due to higher prevalence of infection and reduced access to health care services. On-line events providing vaccination information will be conducted in both English and Spanish.
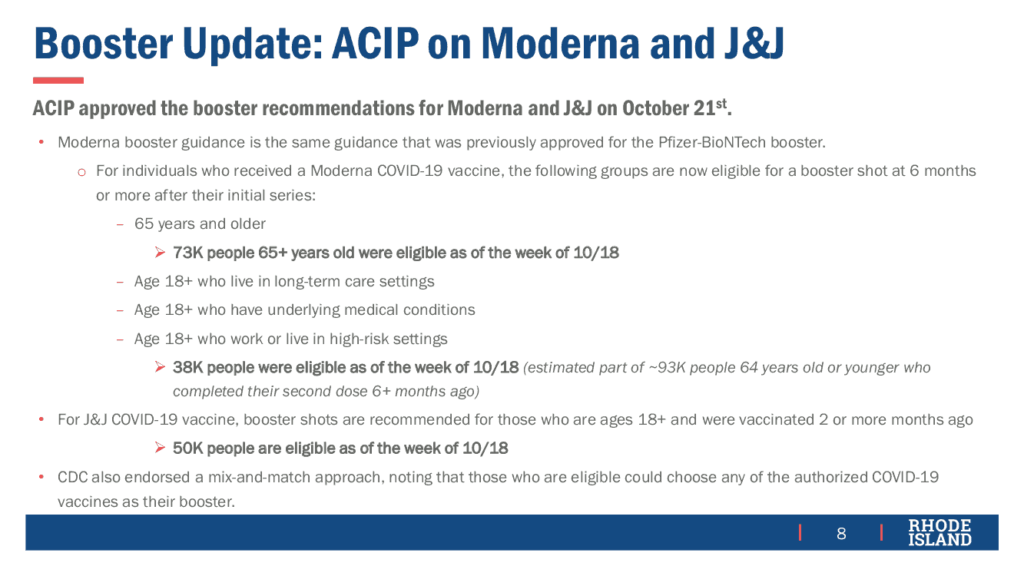
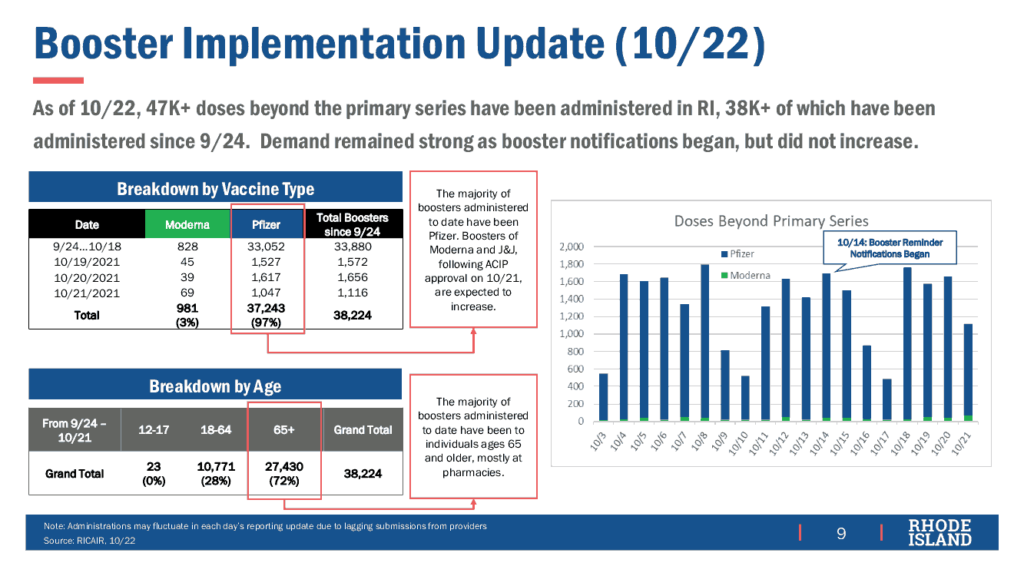
In other news, the Vaccine Sub-Committee was told that, as of October 22, about 47,000 booster doses have been administered in RI with strong demand.
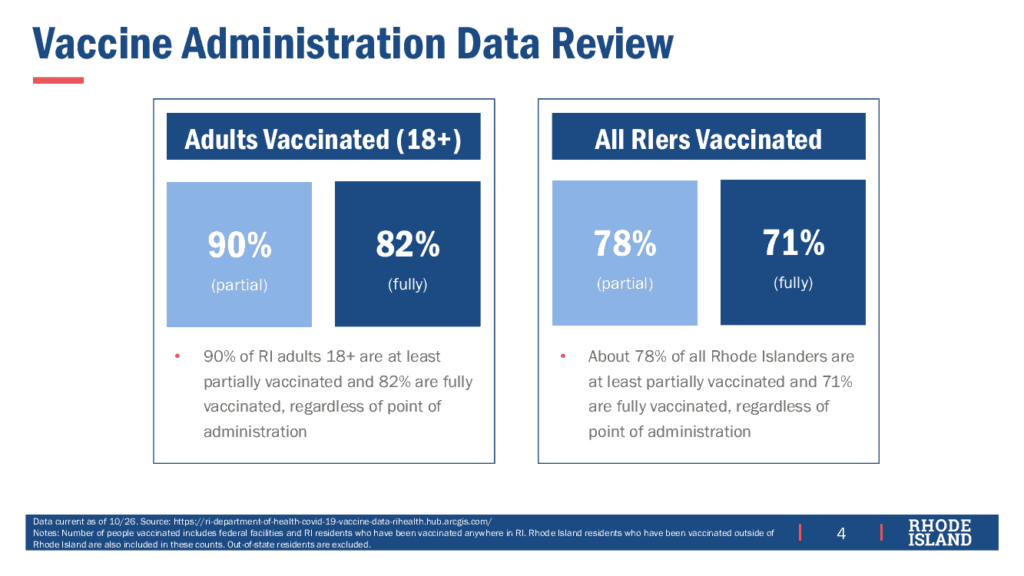
RI has reached 90% partially and 82% fully vaccinated of the population age 18 and older, corresponding to 78% partially and 71% fully vaccinated of the total population.
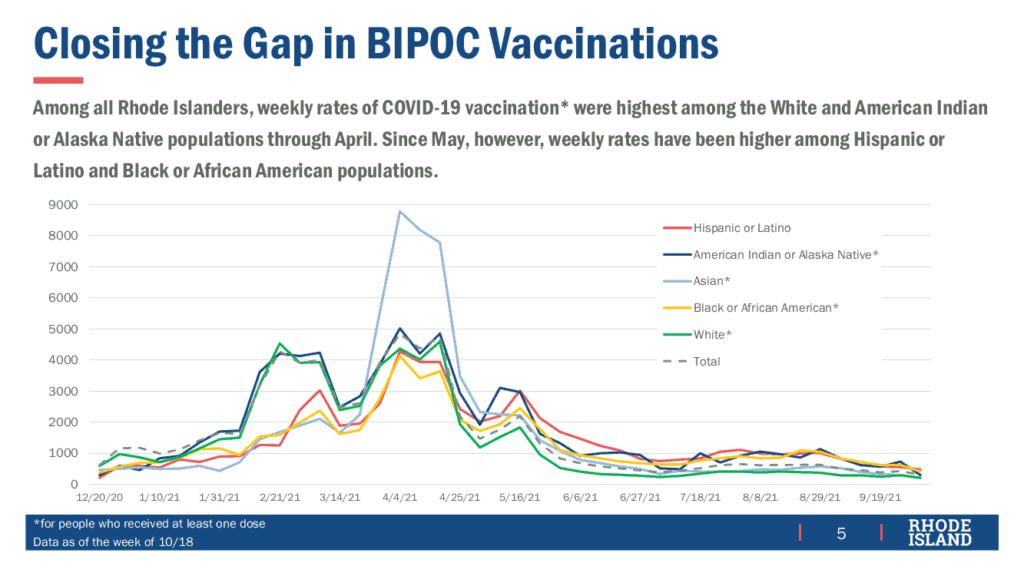
The state continues to invest substantial effort to close the gap in vaccination for those Black, Indigenous, and People of Color (BIPOC) with weekly rates exceeding those for the non-BIPOC population since May, holding more than 700 clinics to address “cold spots” where rates were lower than statistically expected.

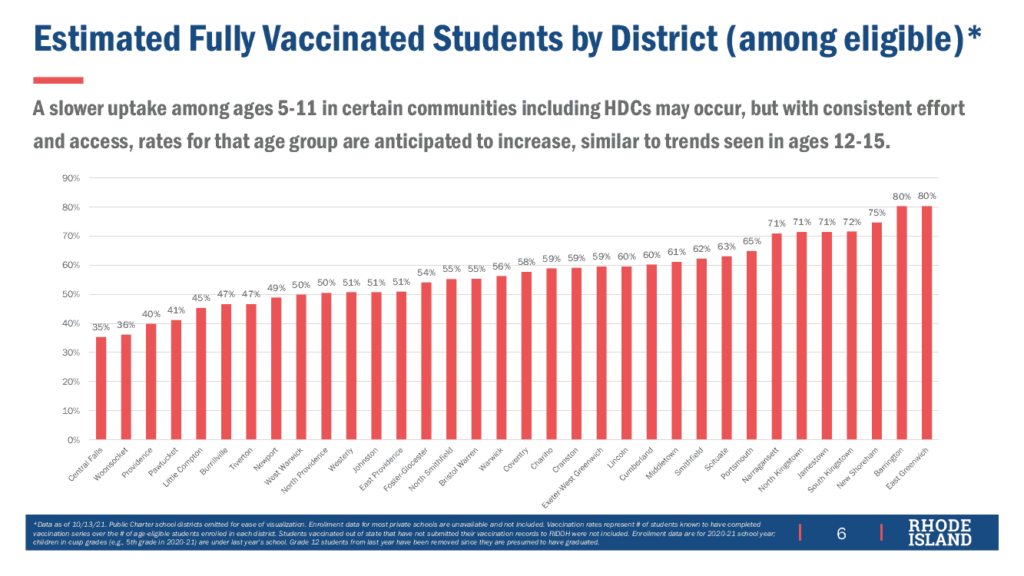
Fully vaccinated rates among non-college students age 12 and older continue to vary enormously by geography, with wealthier municipalities such as Barrington and East Greenwich reaching 80% while poorer ones such as Pawtucket (41%), Providence (40%), Woonsocket (36%), and Central Falls (35%) well below.